DELFIA Neonatal 17α-OH-progesterone kit (10 plate)

DELFIA Neonatal 17α-OH-progesterone kit (10 plate)


| Feature | Specification |
|---|---|
| Application | Newborn Screening |



Product information
Overview
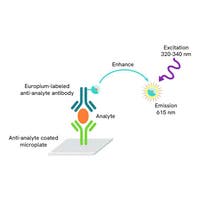
The DELFIA® Neonatal 17α-OH-progesterone (17-OHP) kit is a reliable, high-quality solution for the quantitative determination of 17α-hydroxyprogesterone in dried blood spot samples from newborns. Designed for use with the VICTOR2™ D fluorometric instrument, this kit supports laboratories in delivering accurate and consistent results as part of newborn screening programs.
Why 17α-OH-progesterone?
Measurement of 17-OHP is central to the screening of congenital adrenal hyperplasia (CAH), one of the most common inherited endocrine disorders identified in newborn screening. CAH, typically caused by 21-hydroxylase deficiency, can lead to life-threatening adrenal crises if left undiagnosed. Early identification through newborn screening enables timely medical intervention, reducing the risk of severe complications and supporting improved long-term health outcomes.
Technology you can trust
The DELFIA technology (dissociation-enhanced lanthanide fluorescence immunoassay) combines high sensitivity with robust performance, making it particularly well-suited for routine, high-throughput newborn screening. By using time-resolved fluorometry and europium chelate labels, DELFIA assays provide:
- Excellent signal-to-noise ratio for enhanced sensitivity.
- Low background interference for reliable detection.
- Stable assay performance that ensures consistent, reproducible results.
Workflow efficiency
When integrated with the VICTOR2™ D instrument, the kit supports streamlined workflows in busy newborn screening laboratories. The semi- automated platform allows for efficient assay processing, minimizing manual steps while maintaining high analytical reliability. This ensures laboratories can meet the increasing demands of population-wide screening programs without compromising quality.
Clinical impact
By enabling accurate measurement of 17-OHP in dried blood spots, the DELFIA Neonatal kit empowers screening laboratories and healthcare providers to detect CAH at the earliest possible stage. This early insight is critical to initiating treatment, preventing adrenal crises, and improving the quality of care for affected infants.
A trusted choice in newborn screening
With decades of expertise in neonatal screening solutions, the DELFIA Neonatal 17-OHP kit reflects a commitment to supporting laboratories worldwide with robust, validated tools that make a measurable difference in public health outcomes.
Specifications
| Accreditations |
CE-IVD marked
|
|---|---|
| Application |
Newborn Screening
|
| Brand |
VICTOR2™ D
|
| Detection Modality |
Time-Resolved Fluorescence (TRF)
|
| Disorders |
Congenital Adrenal Hyperplasia (CAH)
|
| Instrument Compatibility |
Victor 2D
|
| Sample Type |
Dried blood spots
|
| Technology |
DELFIA
|
| Unit Size |
10 plates
|
Loading...


Recently viewed

How can we help you?
We are here to answer your questions.