In a compelling and heartfelt presentation at the World View 2024 conference, Vanessa Romanelli shared her extraordinary journey as both a scientist and an individual living with Spinal Muscular Atrophy (SMA). Her talk, titled "Building Tomorrow: The Professional Journey of an SMA Scientist in the Newborn Screening Era," highlighted the intersection of her personal experiences and professional achievements, underscoring the significant advancements in SMA research and newborn screening.
A journey from diagnosis to dedication
Vanessa's story begins in 1985 when she was born, just a few years after Phenylketonuria (PKU) was included in Brazil's newborn screening panel. At the age of five, she began experiencing symptoms of SMA, a motor neuron disease characterized by progressive muscle weakness. A misdiagnosis initially led to unnecessary tests and confusion, but eventually, she was correctly diagnosed with SMA Type 3 through electromyography. This diagnosis came at a time when genetic testing for SMA was not yet available.
It wasn't until she was seven years old that the gene responsible for SMA, SMN1, was discovered. This pivotal moment in genetics ignited Vanessa's passion for the field. Her personal experience with SMA inspired her to pursue a career in genetics, aiming to help others through scientific research.

Watch Vanessa’s World View 2024 conference presentation
Academic and professional milestones
Vanessa's academic journey saw her earning a master's degree and a Ph.D., all the while witnessing significant developments in SMA treatments. In 2016, the first drug for SMA, Spinraza (nusinersen), was approved by the FDA, marking a monumental step forward for the SMA community. This approval coincided with Vanessa's completion of her doctorate, symbolizing the synergy between her personal and professional milestones.
In 2017, Brazil's regulatory agency, Anvisa, approved Spinraza, offering new hope to SMA patients in the country. Vanessa continued her research, focusing on developing and validating genetic tests for SMA, which led to the implementation of pilot studies and expanded newborn screening programs in São Paulo, Brazil.
Innovative treatments and newborn screening
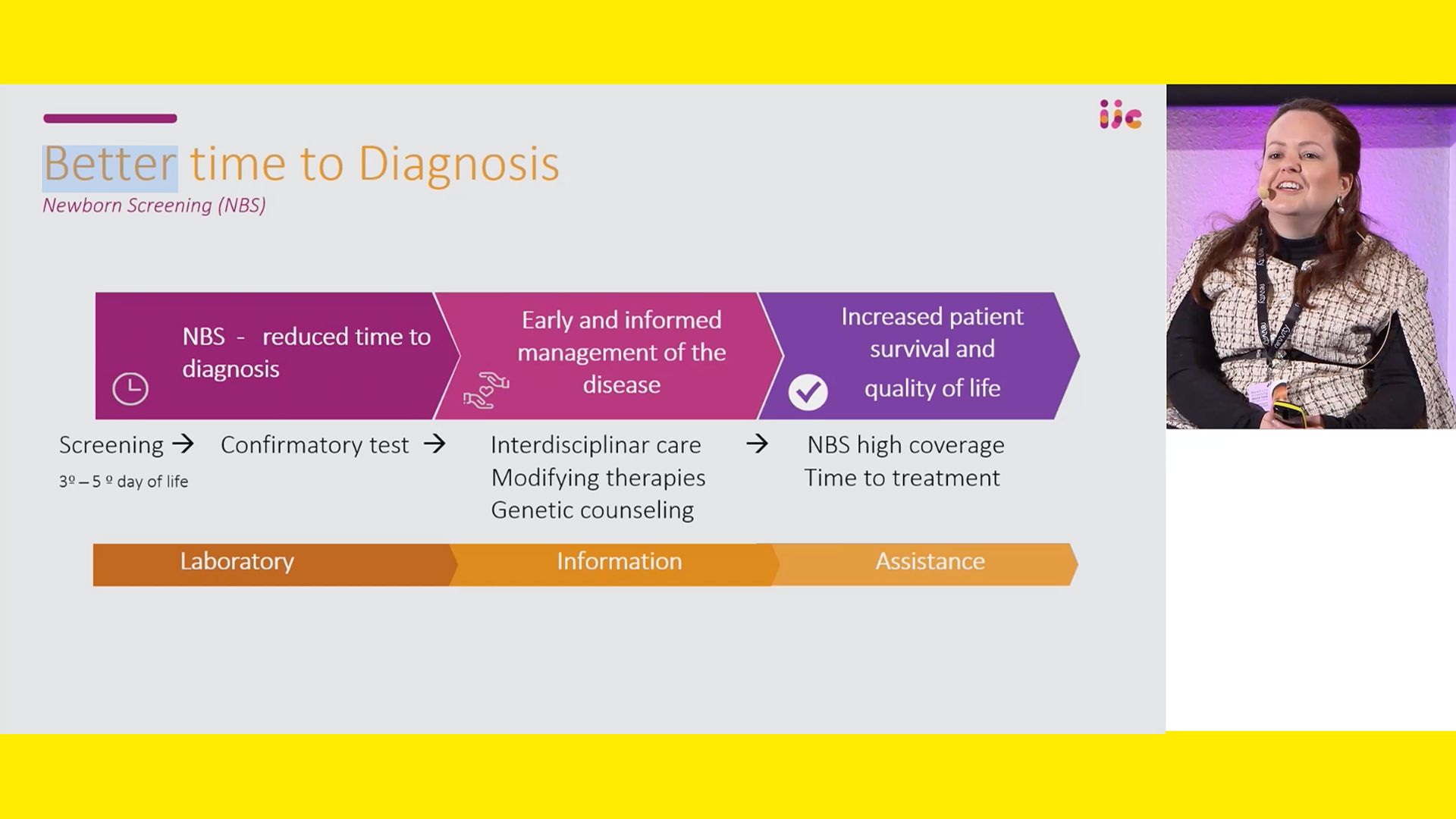
Vanessa provided an overview of the current treatments for SMA, including Spinraza, Zolgensma (onasemnogene abeparvovec), and Risdiplam. These treatments have revolutionized SMA care, particularly when administered pre-symptomatically, as early intervention has shown to significantly improve motor function and reduce the need for permanent ventilation in severe cases.
The success of these treatments has paved the way for SMA to be included in newborn screening panels worldwide. Vanessa highlighted pilot studies and newborn screening programs in various countries, demonstrating the effectiveness of early diagnosis and treatment in improving patient outcomes.
Brazil's newborn screening efforts
In Brazil, Vanessa played a crucial role in validating a genetic test for SMA, which was developed without screening real-world samples initially. Her dedication during the COVID-19 pandemic, with the support of her parents, led to significant progress in this area. The pilot study in São Paulo state, with initiative of Jô Clemente Institute and Romanelli’s coordination, screened thousands of newborns, identifying several SMA cases early and ensuring timely treatment.
Vanessa Romanelli's presentation at the World View conference was a testament to her resilience and dedication to advancing SMA research and newborn screening. Her personal journey from diagnosis to becoming a leading scientist in the field is an inspiring narrative of overcoming challenges and making a meaningful impact.
Her work has not only contributed to the scientific community but also brought hope and improved quality of life to countless individuals with SMA. Vanessa's story exemplifies the power of combining personal experience with professional expertise to drive innovation and change in the healthcare landscape.
Watch Vanessa Romanelli's presentation above and all the presentations from the recent World View conference on demand here: World View 2024 - An International Summit on Newborn Screening.

































